Hydrogen Chloride And Ammonia Reaction
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnesium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | shiny gray solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight A r°(Mg) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnesium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 2 (alkaline earth metals) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Menstruation | period 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | due south-cake | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per beat out | 2, eight, ii | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concrete properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stage atSTP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 923 M (650 °C, 1202 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1363 Yard (1091 °C, 1994 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (nearr.t.) | i.738 yard/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (atyard.p.) | 1.584 1000/cmthree | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 8.48 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oestrus of vaporization | 128 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat chapters | 24.869[ii] J/(mol·Grand) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 0,[iii] +1,[iv] +2 (a strongly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 160 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 141±vii pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 173 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other backdrop | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of audio sparse rod | 4940 g/south (atr.t.) (annealed) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 24.8[5] µm/(m⋅Grand) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 156[6] W/(m⋅Yard) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 43.9[7] nΩ⋅m (at twenty °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tooth magnetic susceptibility | +13.one×10−half dozen cmiii/mol (298 M)[eight] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 45 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 17 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 35.four[9] GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.290 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1–two.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 44–260 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7439-95-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | subsequently Magnesia, Greece[10] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Joseph Black (1755[10]) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Humphry Davy (1808[10]) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Main isotopes of magnesium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Magnesium is a chemical element with the symbolMg and atomic number 12. Information technology is a shiny grayness solid which shares many concrete and chemical backdrop with the other five alkaline earth metals (grouping 2 of the periodic table).
This element is produced in big, crumbling stars from the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it may recycle into new star systems. Magnesium is the eighth most abundant element in the Earth's crust[11] and the quaternary most common chemical element in the Earth (later on iron, oxygen and silicon), making upwardly xiii% of the planet's mass and a large fraction of the planet's pall. It is the 3rd nearly abundant element dissolved in seawater, after sodium and chlorine.[12]
Magnesium occurs naturally but in combination with other elements, where it almost ever has a +2 oxidation land. The gratuitous element (metal) tin can be produced artificially, and is highly reactive (though in the atmosphere it is soon coated in a thin layer of oxide that partly inhibits reactivity – run into passivation). The free metal burns with a feature vivid-white low-cal. The metallic is at present obtained mainly by electrolysis of magnesium salts obtained from brine, and is used primarily as a component in aluminium-magnesium alloys, sometimes called magnalium or magnelium. Magnesium is less dense than aluminium, and the alloy of the ii is prized for its combination of lightness and strength.
This element is the eleventh most abundant chemical element by mass in the human body and is essential to all cells and some 300 enzymes.[13] Magnesium ions collaborate with polyphosphate compounds such as ATP, DNA, and RNA. Hundreds of enzymes require magnesium ions to function. Magnesium compounds are used medicinally as common laxatives, antacids (due east.g., milk of magnesia), and to stabilize abnormal nerve excitation or claret vessel spasm in such conditions equally eclampsia.[13]
Characteristics
Physical properties
Elemental magnesium is a gray-white lightweight metal, two-thirds the density of aluminium. Magnesium has the lowest melting (923 K (650 °C)) and the everyman boiling point 1,363 Yard (ane,090 °C) of all the alkaline earth metals.[xiv]
Pure polycrystalline magnesium is brittle and easily fractures along shear bands. Information technology becomes much more malleable when alloyed with small amount of other metals, such as one% aluminium.[15] The malleability of polycrystalline magnesium can besides be significantly improved by reducing its grain size to ca. 1 micron or less.[sixteen]
When finely powdered, magnesium can react with water to produce hydrogen gas:
- Mg(south) + 2HtwoO(m) → Mg(OH)two(aq) + Hii(g) + 1203.6 kJ
However, this reaction is much less dramatic than the reactions of the alkali metals with water, because the magnesium hydroxide tends to build up on the surface of the pure magnesium metal and prevent the reaction from occurring.[17]
Chemical backdrop
General chemistry
It tarnishes slightly when exposed to air, although, dissimilar the heavier alkaline earth metals, an oxygen-free environment is unnecessary for storage because magnesium is protected past a sparse layer of oxide that is adequately impermeable and difficult to remove.
Straight reaction of magnesium with air or oxygen at ambient pressure level forms just the "normal" oxide MgO. Nonetheless, this oxide may be combined with hydrogen peroxide to course Magnesium peroxide, MgO2, and at low temperature the peroxide may be farther reacted with ozone to form magnesium superoxide Mg(Otwo)two.[eighteen]
Magnesium reacts with water at room temperature, though it reacts much more slowly than calcium, a similar group 2 metal. When submerged in water, hydrogen bubbles class slowly on the surface of the metal – though, if powdered, information technology reacts much more rapidly. The reaction occurs faster with higher temperatures (encounter safety precautions). Magnesium'south reversible reaction with water can be harnessed to store energy and run a magnesium-based engine. Magnesium also reacts exothermically with well-nigh acids such every bit hydrochloric acid (HCl), producing the metal chloride and hydrogen gas, similar to the HCl reaction with aluminium, zinc, and many other metals.
Flammability
Magnesium is highly combustible, especially when powdered or shaved into thin strips, though it is difficult to ignite in mass or bulk. Flame temperatures of magnesium and magnesium alloys can attain three,100 °C (5,610 °F),[nineteen] although flame meridian above the burning metal is usually less than 300 mm (12 in).[20] In one case ignited, such fires are hard to extinguish, because combustion continues in nitrogen (forming magnesium nitride), carbon dioxide (forming magnesium oxide and carbon), and h2o (forming magnesium oxide and hydrogen, which as well combusts due to heat in the presence of additional oxygen). This property was used in incendiary weapons during the firebombing of cities in Globe War Two, where the but applied civil defence force was to smother a burning flare under dry sand to exclude temper from the combustion.
Magnesium may also be used every bit an igniter for thermite, a mixture of aluminium and fe oxide powder that ignites merely at a very loftier temperature.
Organic chemistry
Organomagnesium compounds are widespread in organic chemistry. They are commonly found as Grignard reagents. Magnesium tin can react with haloalkanes to give Grignard reagents. Examples of Grignard reagents are phenylmagnesium bromide and ethylmagnesium bromide. The Grignard reagents role as a common nucleophile, attacking the electrophilic group such every bit the carbon cantlet that is present within the polar bond of a carbonyl group.
A prominent organomagnesium reagent across Grignard reagents is magnesium anthracene with magnesium forming a one,4-bridge over the central ring. It is used every bit a source of highly active magnesium. The related butadiene-magnesium adduct serves as a source for the butadiene dianion.
Magnesium in organic chemistry too appears as low valent magnesium compounds, primarily with the magnesium forming diatomic ions in the +1 oxidation state but more recently also with zip oxidation state or a mixture of +1 and zero states.[21] Such compounds find synthetic application as reducing agents and sources of nucleophilic metal atoms.
Source of light
When burning in air, magnesium produces a brilliant-white light that includes potent ultraviolet wavelengths. Magnesium pulverisation (flash powder) was used for bailiwick illumination in the early days of photography.[22] [23] Later, magnesium filament was used in electrically ignited single-use photography flashbulbs. Magnesium powder is used in fireworks and marine flares where a brilliant white light is required. Information technology was also used for various theatrical furnishings,[24] such as lightning,[25] pistol flashes,[26] and supernatural appearances.[27]
Detection in solution
The presence of magnesium ions can be detected by the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an aqueous or dilute HCl solution of the common salt. The formation of a white precipitate indicates the presence of magnesium ions.
Azo violet dye tin also exist used which turns deep blue in the presence of an alkaline metal solution of magnesium salt. The colour is due to the adsorption of azo violet by Mg(OH)2.
Occurrence
Magnesium is the eighth-most-arable element in the World'southward crust past mass and tied in seventh place with iron in molarity.[11] Information technology is found in large deposits of magnesite, dolomite, and other minerals, and in mineral waters, where magnesium ion is soluble.
Although magnesium is found in more than 60 minerals, only dolomite, magnesite, brucite, carnallite, talc, and olivine are of commercial importance.
The Mg 2+
cation is the second-most-abundant cation in seawater (almost 1⁄8 the mass of sodium ions in a given sample), which makes seawater and ocean table salt bonny commercial sources for Mg. To extract the magnesium, calcium hydroxide is added to seawater to class magnesium hydroxide precipitate.
- MgCl
2 + Ca(OH)
2 → Mg(OH)
2 + CaCl
2
Magnesium hydroxide (brucite) is insoluble in water and tin exist filtered out and reacted with hydrochloric acrid to produced concentrated magnesium chloride.
- Mg(OH)
2 + 2 HCl → MgCl
ii + two H
2 O
From magnesium chloride, electrolysis produces magnesium.
Forms
Alloys
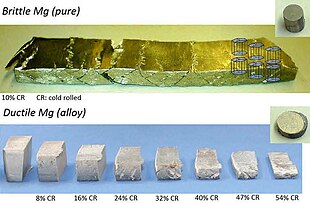
Magnesium is brittle, and fractures forth shear bands when its thickness is reduced by but 10% by cold rolling (height). However, after alloying Mg with one% Al and 0.1% Ca, its thickness could be reduced by 54% using the same procedure (bottom).
As of 2013, magnesium alloys consumption was less than one million tonnes per year, compared with 50 million tonnes of aluminum alloys. Their use has been historically limited by the tendency of Mg alloys to corrode,[28] creep at high temperatures, and combust.[29]
Corrosion
The presence of iron, nickel, copper, and cobalt strongly activates corrosion. In more trace amounts, these metals precipitate as intermetallic compounds, and the precipitate locales office equally active cathodic sites that reduce h2o, causing the loss of magnesium.[29] Decision-making the quantity of these metals improves corrosion resistance. Sufficient manganese overcomes the corrosive furnishings of iron. This requires precise control over composition, increasing costs.[29] Adding a cathodic poison captures atomic hydrogen inside the structure of a metal. This prevents the formation of complimentary hydrogen gas, an essential factor of corrosive chemical processes. The addition of virtually i in 3 hundred parts arsenic reduces its corrosion rate in a salt solution by a gene of nearly ten.[29] [30]
High-temperature creep and flammability
Research showed that magnesium's tendency to creep at high temperatures is eliminated by the addition of scandium and gadolinium. Flammability is profoundly reduced by a minor amount of calcium in the alloy.[29] By using rare-globe elements, information technology may be possible to industry magnesium alloys with an ignition temperature higher than magnesium'south liquidus and in some cases potentially pushing it close to magnesium's boiling betoken.[31]
Compounds
Magnesium forms a multifariousness of compounds important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide (milk of magnesia), magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate (Epsom salts).
Isotopes
Magnesium has three stable isotopes: 24
Mg, 25
Mg and 26
Mg. All are present in meaning amounts in nature (see table of isotopes in a higher place). Nearly 79% of Mg is 24
Mg. The isotope 28
Mg is radioactive and in the 1950s to 1970s was produced by several nuclear power plants for use in scientific experiments. This isotope has a relatively short half-life (21 hours) and its utilise was limited by aircraft times.
The nuclide 26
Mg has found awarding in isotopic geology, like to that of aluminium. 26
Mg is a radiogenic daughter product of 26
Al, which has a half-life of 717,000 years. Excessive quantities of stable 26
Mg take been observed in the Ca-Al-rich inclusions of some carbonaceous chondrite meteorites. This anomalous affluence is attributed to the decay of its parent 26
Al in the inclusions, and researchers conclude that such meteorites were formed in the solar nebula before the 26
Al had decayed. These are among the oldest objects in the Solar Arrangement and contain preserved data about its early history.
It is conventional to plot 26
Mg/ 24
Mg against an Al/Mg ratio. In an isochron dating plot, the Al/Mg ratio plotted is 27
Al/ 24
Mg. The slope of the isochron has no age significance, only indicates the initial 26
Al/ 27
Al ratio in the sample at the time when the systems were separated from a common reservoir.
Production

Magnesium sheets and ingots
Globe production was approximately 1,100 kt in 2017, with the bulk existence produced in Cathay (930 kt) and Russia (60 kt).[32] The U.s. was in the 20th century the major world supplier of this metal, supplying 45% of world production fifty-fifty every bit recently as 1995. Since the Chinese mastery of the Pidgeon process the US market share is at vii%, with a single US producer left: US Magnesium, a Renco Group visitor in Utah born from now-defunct Magcorp.[33]
In September 2021, China took steps to reduce production of magnesium as a outcome of a government initiative to reduce energy availability for manufacturing industries, leading to a significant cost increase.[34]
- Pidgeon procedure
China is almost completely reliant on the silicothermic Pidgeon process (the reduction of the oxide at high temperatures with silicon, frequently provided by a ferrosilicon alloy in which the iron is but a spectator in the reactions) to obtain the metallic.[35] The process tin can also exist carried out with carbon at approx 2300 °C:
- 2MgO
(s) + Si
(s) + 2CaO
(southward) → 2Mg
(m) + Ca
2 SiO
4(south) - MgO
(s) + C
(s) → Mg
(g) + CO
(g)
- Dow process
In the United States, magnesium is obtained principally with the Dow process, past electrolysis of fused magnesium chloride from alkali and body of water water. A saline solution containing Mg two+
ions is outset treated with lime (calcium oxide) and the precipitated magnesium hydroxide is collected:
- Mg 2+
(aq) + CaO
(south) + H
2 O → Ca 2+
(aq) + Mg(OH)
2(s)
The hydroxide is then converted to a fractional hydrate of magnesium chloride by treating the hydroxide with muriatic acid and heating of the production:
- Mg(OH)
2(south) + 2 HCl → MgCl
2(aq) + iiH
two O
(l)
The salt is then electrolyzed in the molten state. At the cathode, the Mg 2+
ion is reduced past two electrons to magnesium metal:
- Mg 2+
+ 2
e −
→ Mg
At the anode, each pair of Cl −
ions is oxidized to chlorine gas, releasing two electrons to complete the circuit:
- 2 Cl −
→ Cl
2 (yard) + 2
e −
- YSZ process
A new process, solid oxide membrane technology, involves the electrolytic reduction of MgO. At the cathode, Mg 2+
ion is reduced by two electrons to magnesium metal. The electrolyte is yttria-stabilized zirconia (YSZ). The anode is a liquid metal. At the YSZ/liquid metal anode O two−
is oxidized. A layer of graphite borders the liquid metallic anode, and at this interface carbon and oxygen react to form carbon monoxide. When silverish is used as the liquid metal anode, in that location is no reductant carbon or hydrogen needed, and just oxygen gas is evolved at the anode.[36] Information technology has been reported that this method provides a xl% reduction in cost per pound over the electrolytic reduction method.[37]
History
The proper name magnesium originates from the Greek word for locations related to the tribe of the Magnetes, either a commune in Thessaly chosen Magnesia[38] or Magnesia ad Sipylum, now in Turkey.[39] It is related to magnetite and manganese, which also originated from this area, and required differentiation as separate substances. See manganese for this history.
In 1618, a farmer at Epsom in England attempted to give his cows water from a well in that location. The cows refused to drink because of the h2o'due south bitter taste, just the farmer noticed that the water seemed to heal scratches and rashes. The substance became known as Epsom salts and its fame spread.[40] It was somewhen recognized every bit hydrated magnesium sulfate, MgSO
4 ·7H
2 O.
The metallic itself was first isolated by Sir Humphry Davy in England in 1808. He used electrolysis on a mixture of magnesia and mercuric oxide.[41] Antoine Bussy prepared information technology in coherent course in 1831. Davy'due south outset suggestion for a name was magnium,[41] simply the name magnesium is now used in English and all major European languages merely Russian.
Uses as a metallic

Magnesium is the tertiary-most-commonly-used structural metal, following iron and aluminium.[42] The main applications of magnesium are, in lodge: aluminium alloys, die-casting (alloyed with zinc),[43] removing sulfur in the production of iron and steel, and the production of titanium in the Kroll process.[44]
Magnesium is used in lightweight materials and alloys. For case, when infused with silicon carbide nanoparticles, information technology has extremely loftier specific strength.[45]
Historically, magnesium was one of the main aerospace structure metals and was used for High german military aircraft as early equally World War I and extensively for German language aircraft in World War II. The Germans coined the name "Elektron" for magnesium alloy, a term which is however used today. In the commercial aerospace industry, magnesium was generally restricted to engine-related components, due to fire and corrosion hazards. Magnesium blend apply in aerospace is increasing in the 21st century, driven by the importance of fuel economy.[46] Evolution and testing of new magnesium alloys continues, notably Elektron 21, which (in test) has proved suitable for aerospace engine, internal, and airframe components.[47] The European Customs runs iii R&D magnesium projects in the Aerospace priority of the FP6 Program. Recent developments in metallurgy and manufacturing have immune for the potential for magnesium alloys to act as replacements for aluminium and steel alloys in certain applications.[48] [49]
In the form of thin ribbons, magnesium is used to purify solvents; for instance, preparing super-dry out ethanol.[ citation needed ]
Aircraft
- Wright Aeronautical used a magnesium crankcase in the WWII-era Wright R-3350 Duplex Cyclone aviation engine. This presented a serious problem for the earliest models of the Boeing B-29 Superfortress heavy bomber when an in-flight engine burn ignited the engine crankcase. The resulting combustion was as hot as 5,600 °F (iii,100 °C) and could sever the wing spar from the fuselage.[50] [51] [52]
Automotive

Mg alloy motorbike engine blocks
- Mercedes-Benz used the alloy Elektron in the bodywork of an early model Mercedes-Benz 300 SLR; these cars competed in the 1955 World Sportscar Title including a win at the Mille Miglia, and at Le Mans where one was involved in the 1955 Le Mans disaster when spectators were showered with burning fragments of elektron.[ citation needed ]
- Porsche used magnesium blend frames in the 917/053 that won Le Mans in 1971, and continues to use magnesium alloys for its engine blocks due to the weight reward.[ citation needed ]
- Volkswagen Grouping has used magnesium in its engine components for many years.[53]
- Mitsubishi Motors uses magnesium for its paddle shifters.[ citation needed ]
- BMW used magnesium alloy blocks in their N52 engine, including an aluminium alloy insert for the cylinder walls and cooling jackets surrounded by a high-temperature magnesium alloy AJ62A. The engine was used worldwide between 2005 and 2011 in diverse one, 3, 5, 6, and vii serial models; likewise equally the Z4, X1, X3, and X5.[ citation needed ]
- Chevrolet used the magnesium blend AE44 in the 2006 Corvette Z06.[ commendation needed ]
Both AJ62A and AE44 are contempo developments in high-temperature depression-creep magnesium alloys. The general strategy for such alloys is to course intermetallic precipitates at the grain boundaries, for example by adding mischmetal or calcium.[54] New alloy development and lower costs that make magnesium competitive with aluminium volition increase the number of automotive applications.[ commendation needed ]
Electronics
Considering of depression density and adept mechanical and electrical properties, magnesium is used for manufacturing of mobile phones, laptop and tablet computers, cameras, and other electronic components.[ citation needed ] It was used as a premium feature considering of its low-cal weight in some 2020 laptops.[55]

Products made of magnesium: firestarter and shavings, sharpener, magnesium ribbon
Other
Magnesium, existence readily bachelor and relatively nontoxic, has a diverseness of uses:
- Magnesium is combustible, burning at a temperature of approximately iii,100 °C (3,370 K; 5,610 °F),[19] and the autoignition temperature of magnesium ribbon is approximately 473 °C (746 Thousand; 883 °F).[56] It produces intense, vivid, white low-cal when it burns. Magnesium's high combustion temperature makes it a useful tool for starting emergency fires. Other uses include flash photography, flares, pyrotechnics, fireworks sparklers, and trick birthday candles. Magnesium is besides oft used to ignite thermite or other materials that require a high ignition temperature.

Magnesium firestarter (in left manus), used with a pocket knife and flint to create sparks that ignite the shavings
- In the form of turnings or ribbons, to prepare Grignard reagents, which are useful in organic synthesis.[ citation needed ]
- As an additive agent in conventional propellants and the production of nodular graphite in cast fe.[ citation needed ]
- Every bit a reducing amanuensis to separate uranium and other metals from their salts.[ citation needed ]
- As a sacrificial (galvanic) anode to protect boats, undercover tanks, pipelines, buried structures, and water heaters.[ citation needed ]
- Alloyed with zinc to produce the zinc canvass used in photoengraving plates in the press industry, dry-cell battery walls, and covering.[43]
- As a metallic, this element'southward principal utilize is as an alloying additive to aluminium with these aluminium-magnesium alloys existence used mainly for potable cans, sports equipment such every bit golf clubs, angling reels, and archery bows and arrows.[ commendation needed ]
- Specialty, high-grade car wheels of magnesium alloy are called "magazine wheels", although the term is often misapplied to aluminium wheels. Many car and aircraft manufacturers have made engine and trunk parts from magnesium.[ commendation needed ]
- Magnesium batteries have been commercialized equally primary batteries, and are an active topic of inquiry for rechargeable batteries.[ commendation needed ]
Safety precautions
Magnesium block heated with blowtorch to self-combustion, emitting intense white low-cal
| Hazards | |
|---|---|
| GHS labelling: | |
| Pictograms |  |
| Signal discussion | Danger |
| Hazard statements | H228, H251, H261 |
| Precautionary statements | P210, P231, P235, P410, P422 [57] |
| NFPA 704 (fire diamond) | https://cameochemicals.noaa.gov/chemic/6949 0 i one |
Magnesium metallic and its alloys can be explosive hazards; they are highly flammable in their pure grade when molten or in powder or ribbon form. Burning or molten magnesium reacts violently with water. When working with powdered magnesium, safety spectacles with eye protection and UV filters (such as welders use) are employed considering burning magnesium produces ultraviolet lite that tin permanently damage the retina of a human middle.[58]
Magnesium is capable of reducing water and releasing highly flammable hydrogen gas:[59]
- Mg (s) + two H
2 O (fifty) → Mg(OH)
2 (s) + H
2 (chiliad)
Therefore, water cannot extinguish magnesium fires. The hydrogen gas produced intensifies the fire. Dry out sand is an effective smothering agent, merely but on relatively level and flat surfaces.
Magnesium reacts with carbon dioxide exothermically to form magnesium oxide and carbon:[threescore]
- 2 Mg + CO
2 → 2 MgO + C (south)
Hence, carbon dioxide fuels rather than extinguishes magnesium fires.
Burning magnesium tin be quenched by using a Class D dry chemic fire extinguisher, or by covering the burn down with sand or magnesium foundry flux to remove its air source.[61]
Useful compounds
Magnesium compounds, primarily magnesium oxide (MgO), are used as a refractory material in furnace linings for producing iron, steel, nonferrous metals, drinking glass, and cement. Magnesium oxide and other magnesium compounds are too used in the agricultural, chemical, and structure industries. Magnesium oxide from calcination is used as an electrical insulator in burn-resistant cables.[62]
Magnesium hydride is under investigation as a mode to shop hydrogen.
Magnesium reacted with an alkyl halide gives a Grignard reagent, which is a very useful tool for preparing alcohols.
Magnesium salts are included in various foods, fertilizers (magnesium is a component of chlorophyll), and microbe culture media.
Magnesium sulfite is used in the manufacture of newspaper (sulfite process).
Magnesium phosphate is used to fireproof wood used in structure.
Magnesium hexafluorosilicate is used for moth-proofing textiles.
Biological roles
Mechanism of action
The of import interaction between phosphate and magnesium ions makes magnesium essential to the basic nucleic acid chemical science of all cells of all known living organisms. More than 300 enzymes require magnesium ions for their catalytic activity, including all enzymes using or synthesizing ATP and those that employ other nucleotides to synthesize Dna and RNA. The ATP molecule is normally found in a chelate with a magnesium ion.[63]
Diet
Diet

Examples of food sources of magnesium (clockwise from top left): bran muffins, pumpkin seeds, barley, buckwheat flour, low-fat vanilla yogurt, trail mix, halibut steaks, garbanzo beans, lima beans, soybeans, and spinach
Spices, nuts, cereals, cocoa and vegetables are rich sources of magnesium.[thirteen] Green leafy vegetables such every bit spinach are also rich in magnesium.[64]
Beverages rich in magnesium are coffee, tea, and cocoa.[65]
Dietary recommendations
In the UK, the recommended daily values for magnesium are 300 mg for men and 270 mg for women.[66] In the U.Southward. the Recommended Dietary Allowances (RDAs) are 400 mg for men ages 19–30 and 420 mg for older; for women 310 mg for ages 19–30 and 320 mg for older.[67]
Supplementation
Numerous pharmaceutical preparations of magnesium and dietary supplements are available. In two human trials magnesium oxide, i of the virtually mutual forms in magnesium dietary supplements because of its high magnesium content per weight, was less bioavailable than magnesium citrate, chloride, lactate or aspartate.[68] [69]
Metabolism
An adult body has 22–26 grams of magnesium,[xiii] [70] with 60% in the skeleton, 39% intracellular (twenty% in skeletal muscle), and 1% extracellular.[13] Serum levels are typically 0.seven–1.0 mmol/L or one.eight–2.4 mEq/50. Serum magnesium levels may be normal even when intracellular magnesium is deficient. The mechanisms for maintaining the magnesium level in the serum are varying gastrointestinal assimilation and renal excretion. Intracellular magnesium is correlated with intracellular potassium. Increased magnesium lowers calcium[71] and can either prevent hypercalcemia or cause hypocalcemia depending on the initial level.[71] Both depression and high poly peptide intake atmospheric condition inhibit magnesium absorption, as does the amount of phosphate, phytate, and fat in the gut. Unabsorbed dietary magnesium is excreted in feces; absorbed magnesium is excreted in urine and sweat.[72]
Detection in serum and plasma
Magnesium condition may be assessed by measuring serum and erythrocyte magnesium concentrations coupled with urinary and fecal magnesium content, but intravenous magnesium loading tests are more accurate and practical.[73] A retention of 20% or more of the injected amount indicates deficiency.[74] As of 2004, no biomarker has been established for magnesium.[75]
Magnesium concentrations in plasma or serum may be monitored for efficacy and safety in those receiving the drug therapeutically, to confirm the diagnosis in potential poisoning victims, or to assist in the forensic investigation in a case of fatal overdose. The newborn children of mothers who received parenteral magnesium sulfate during labor may exhibit toxicity with normal serum magnesium levels.[76]
Deficiency
Low plasma magnesium (hypomagnesemia) is mutual: information technology is found in 2.5–15% of the general population.[77] From 2005 to 2006, 48 percent of the The states population consumed less magnesium than recommended in the Dietary Reference Intake.[78] Other causes are increased renal or gastrointestinal loss, an increased intracellular shift, and proton-pump inhibitor antacid therapy. Most are asymptomatic, but symptoms referable to neuromuscular, cardiovascular, and metabolic dysfunction may occur.[77] Alcoholism is often associated with magnesium deficiency. Chronically low serum magnesium levels are associated with metabolic syndrome, diabetes mellitus type ii, fasciculation, and hypertension.[79]
Therapy
- Intravenous magnesium is recommended past the ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Expiry for patients with ventricular arrhythmia associated with torsades de pointes who present with long QT syndrome; and for the treatment of patients with digoxin induced arrhythmias.[eighty]
- Magnesium sulfate – intravenous – is used for the management of pre-eclampsia and eclampsia.[81] [82]
- Hypomagnesemia, including that acquired by alcoholism, is reversible by oral or parenteral magnesium administration depending on the caste of deficiency.[83]
- In that location is express show that magnesium supplementation may play a function in the prevention and treatment of migraine.[84]
Sorted by type of magnesium salt, other therapeutic applications include:
- Magnesium sulfate, as the heptahydrate called Epsom salts, is used as bath salts, a laxative, and a highly soluble fertilizer.[85]
- Magnesium hydroxide, suspended in h2o, is used in milk of magnesia antacids and laxatives.
- Magnesium chloride, oxide, gluconate, malate, orotate, glycinate, ascorbate and citrate are all used as oral magnesium supplements.
- Magnesium borate, magnesium salicylate, and magnesium sulfate are used as antiseptics.
- Magnesium bromide is used equally a mild allaying (this activeness is due to the bromide, non the magnesium).
- Magnesium stearate is a slightly flammable white powder with lubricating properties. In pharmaceutical engineering science, it is used in pharmacological manufacture to foreclose tablets from sticking to the equipment while compressing the ingredients into tablet form.
- Magnesium carbonate pulverization is used by athletes such as gymnasts, weightlifters, and climbers to eliminate palm sweat, forbid sticking, and improve the grip on gymnastic apparatus, lifting confined, and climbing rocks.
Overdose
Overdose from dietary sources solitary is unlikely because excess magnesium in the blood is promptly filtered by the kidneys,[77] and overdose is more likely in the presence of impaired renal function. In spite of this, megadose therapy has caused decease in a young child,[86] and severe hypermagnesemia in a woman[87] and a young daughter[88] who had good for you kidneys. The almost mutual symptoms of overdose are nausea, vomiting, and diarrhea; other symptoms include hypotension, defoliation, slowed heart and respiratory rates, deficiencies of other minerals, coma, cardiac arrhythmia, and death from cardiac arrest.[71]
Role in plants
Plants require magnesium to synthesize chlorophyll, essential for photosynthesis. Magnesium in the center of the porphyrin band in chlorophyll functions in a style similar to the atomic number 26 in the centre of the porphyrin ring in heme. Magnesium deficiency in plants causes late-flavour yellowing between foliage veins, especially in older leaves, and tin be corrected by either applying epsom salts (which is rapidly leached), or crushed dolomitic limestone, to the soil.
Meet also
- List of countries by magnesium production
- Magnesium oil
References
- ^ "Standard Diminutive Weights: Magnesium". CIAAW. 2011.
- ^ Rumble, p. 4.61
- ^ Mg(0) has been synthesized in a chemical compound containing a NaiiMg2 2+ cluster coordinated to a bulky organic ligand; see Rösch, B.; Gentner, T. X.; Eyselein, J.; Langer, J.; Elsen, H.; Li, W.; Harder, Southward. (2021). "Strongly reducing magnesium(0) complexes". Nature. 592 (7856): 717–721. Bibcode:2021Natur.592..717R. doi:ten.1038/s41586-021-03401-westward. PMID 33911274. S2CID 233447380
- ^ Bernath, P. F.; Blackness, J. H. & Brault, J. W. (1985). "The spectrum of magnesium hydride" (PDF). Astrophysical Journal. 298: 375. Bibcode:1985ApJ...298..375B. doi:x.1086/163620. . See likewise Depression valent magnesium compounds.
- ^ Rumble, p. 12.135
- ^ Rumble, p. 12.137
- ^ Rumble, p. 12.28
- ^ Rumble, p. four.lxx
- ^ Gschneider, K. A. (1964). Physical Properties and Interrelationships of Metallic and Semimetallic Elements. Solid State Physics. Vol. 16. p. 308. doi:10.1016/S0081-1947(08)60518-4. ISBN9780126077162.
- ^ a b c Rumble, p. four.19
- ^ a b "Affluence and form of the almost abundant elements in Earth's continental crust" (PDF). Archived from the original (PDF) on 27 September 2011. Retrieved xv February 2008.
- ^ Anthoni, J Floor (2006). "The chemical limerick of seawater". seafriends.org.nz.
- ^ a b c d due east "Dietary Supplement Fact Sheet: Magnesium". Office of Dietary Supplements, U.s. National Institutes of Wellness. eleven Feb 2016. Retrieved 13 October 2016.
- ^ "alkaline-earth metal - Physical and chemical behaviour | Britannica". www.britannica.com . Retrieved 27 March 2022.
- ^ Sandlöbes, Due south.; Friák, G.; Korte-Kerzel, Southward.; Pei, Z.; Neugebauer, J.; Raabe, D. (2017). "A rare-globe gratis magnesium alloy with improved intrinsic ductility". Scientific Reports. 7 (1): 10458. Bibcode:2017NatSR...710458S. doi:10.1038/s41598-017-10384-0. PMC5585333. PMID 28874798.
- ^ Zeng, Zhuoran; Nie, Jian-Feng; Xu, Shi-Wei; h. j. Davies, Chris; Birbilis, Nick (2017). "Super-formable pure magnesium at room temperature". Nature Communications. 8 (1): 972. Bibcode:2017NatCo...8..972Z. doi:10.1038/s41467-017-01330-9. PMC5715137. PMID 29042555.
- ^ "Reactions of Group 2 Elements with Water". Chemistry LibreTexts. 3 October 2013. Retrieved 27 March 2022.
- ^ Vol'nov, I.I., Tokareva, Due south.A., Belevskii, V.N. et al. "The germination of magnesium perperoxide Mg(O2)2 in the reaction of magnesium peroxide with ozone" _Russ Chem Bull_ **19**, 468–471 (1970). https://doi.org/ten.1007/BF00848959
- ^ a b Dreizin, Edward L.; Berman, Charles H. & Vicenzi, Edward P. (2000). "Condensed-stage modifications in magnesium particle combustion in air". Scripta Materialia. 122 (1–ii): 30–42. CiteSeerXten.1.1.488.2456. doi:10.1016/S0010-2180(00)00101-ii.
- ^ DOE Handbook – Primer on Spontaneous Heating and Pyrophoricity. U.S. Section of Energy. December 1994. p. twenty. DOE-HDBK-1081-94. Archived from the original on 15 April 2012. Retrieved 21 Dec 2011.
- ^ B. Rösch, T. X. Gentner, J. Eyselein, J. Langer, H. Elsen & S. Harder, "Strongly reducing magnesium(0) complexes", _Nature_ **592**, 717–721 (2021). [doi: 10.1038/s41586-021-03401-due west](https://doi.org/10.1038/s41586-021-03401-w)
- ^ Hannavy, John (2013). Encyclopedia of Nineteenth-Century Photography. Routledge. p. 84. ISBN978-1135873271.
- ^ Scientific American: Supplement. Vol. 48. Munn and Company. 1899. p. 20035.
- ^ Billboard. Nielsen Business organisation Media, Inc. 1974. p. 20.
- ^ Altman, Rick (2007). Silent Flick Sound. Columbia University Press. p. 41. ISBN978-0231116633.
- ^ Lindsay, David (2005). Madness in the Making: The Triumphant Rise & Untimely Fall of America'south Show Inventors. iUniverse. p. 210. ISBN978-0595347667.
- ^ McCormick, John; Pratasik, Bennie (2005). Popular Boob Theatre in Europe, 1800–1914. Cambridge University Printing. p. 106. ISBN978-0521616157.
- ^ Makar, 1000. L.; Kruger, J. (1993). "Corrosion of magnesium". International Materials Reviews. 38 (3): 138–153. doi:10.1179/imr.1993.38.three.138.
- ^ a b c d e Dodson, Brian (29 August 2013). "Stainless magnesium breakthrough bodes well for manufacturing industries". Gizmag.com. Retrieved 29 August 2013.
- ^ Birbilis, N.; Williams, Thou.; Gusieva, 1000.; Samaniego, A.; Gibson, M. A.; McMurray, H. North. (2013). "Poisoning the corrosion of magnesium". Electrochemistry Communications. 34: 295–298. doi:10.1016/j.elecom.2013.07.021.
- ^ Czerwinski, Frank. "Controlling the ignition and flammability of magnesium for aerospace applications." Corrosion Scientific discipline 86 (2014): ane-16.
- ^ Bray, E. Lee (Feb 2019) Magnesium Metal. Mineral Commodity Summaries, U.South. Geological Survey
- ^ Vardi, Nathan. "Human being With Many Enemies". Forbes . Retrieved 30 January 2021.
- ^ What to exercise about the magnesium shortage, Supply Direction, published 17 February 2022, accessed 12 June 2022
- ^ "Magnesium Overview". China magnesium Corporation. Retrieved eight May 2013.
- ^ Pal, Uday B.; Powell, Adam C. (2007). "The Apply of Solid-Oxide-Membrane Technology for Electrometallurgy". JOM. 59 (5): 44–49. Bibcode:2007JOM....59e..44P. doi:10.1007/s11837-007-0064-x. S2CID 97971162.
- ^ Derezinski, Steve (12 May 2011). "Solid Oxide Membrane (SOM) Electrolysis of Magnesium: Scale-Up Inquiry and Engineering for Light-Weight Vehicles" (PDF). MOxST. Archived from the original (PDF) on thirteen November 2013. Retrieved 27 May 2013.
- ^ "Magnesium: historical information". webelements.com. Retrieved 9 Oct 2014.
- ^ languagehat (28 May 2005). "MAGNET". languagehat.com . Retrieved 18 June 2020.
- ^ Ainsworth, Steve (1 June 2013). "Epsom'due south deep bath". Nurse Prescribing. eleven (6): 269. doi:10.12968/npre.2013.11.vi.269.
- ^ a b Davy, H. (1808). "Electro-chemical researches on the decomposition of the earths; with observations on the metals obtained from the alkaline metal earths, and on the amalgam procured from ammonia". Philosophical Transactions of the Majestic Society of London. 98: 333–370. Bibcode:1808RSPT...98..333D. doi:ten.1098/rstl.1808.0023. JSTOR 107302.
- ^ Segal, David (2017). Materials for the 21st Century. Oxford University Press. ISBN978-0192526090.
- ^ a b Bakery, Hugh D. R.; Avedesian, Michael (1999). Magnesium and magnesium alloys. Materials Park, OH: Materials Information Club. p. 4. ISBN978-0871706577.
- ^ Ketil Amundsen; Terje Kr. Aune; Per Bakke; Hans R. Eklund; Johanna Ö. Haagensen; Carlos Nicolas; et al. (2002). "Magnesium". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:ten.1002/14356007.a15_559. ISBN978-3527306732.
- ^ Chin, Matthew (23 Dec 2015). "UCLA researchers create super-strong magnesium metal". ucla.edu.
- ^ Aghion, E.; Bronfin, B. (2000). "Magnesium Alloys Development towards the 21st Century". Materials Science Forum. 350–351: xix–30. doi:ten.4028/world wide web.scientific.net/MSF.350-351.19. S2CID 138429749.
- ^ Bronfin, B.; et al. (2007). "Elektron 21 specification". In Kainer, Karl (ed.). Magnesium: Proceedings of the 7th International Conference on Magnesium Alloys and Their Applications. Weinheim, Frg: Wiley. p. 23. ISBN978-3527317646.
- ^ Shu, Dong Wei, and Iram Raza Ahmad. "Magnesium Alloys: An Culling for Aluminium in Structural Applications." In Avant-garde Materials Research, vol. 168, pp. 1631-1635. Trans Tech Publications Ltd, 2011.
- ^ Magnesium alloy equally a lighter alternative to aluminum alloy, Phys.org, Nov 29th 2017
- ^ Dreizin, Edward L.; Berman, Charles H.; Vicenzi, Edward P. (2000). "Condensed-phase modifications in magnesium particle combustion in air". Scripta Materialia. 122 (1–2): 30–42. CiteSeerX10.1.1.488.2456. doi:10.1016/S0010-2180(00)00101-2.
- ^ Dorr, Robert F. (fifteen September 2012). Mission to Tokyo: The American Airmen Who Took the War to the Center of Japan. pp. xl–41. ISBN978-1610586634.
- ^ AAHS Journal. Vol. 44–45. American Aviation Historical Society. 1999.
- ^ "1950: The metal is magnesium, the car is the Protrude". www.hydro.com. 18 August 2020. Retrieved five April 2021.
- ^ Luo, Alan A. & Powell, Bob R. (2001). "Tensile and Compressive Creep of Magnesium-Aluminum-Calcium Based Alloys" (PDF). Materials & Processes Laboratory, Full general Motors Research & Development Eye. Archived from the original (PDF) on 28 September 2007. Retrieved 21 August 2007.
- ^ Dignan, Larry (two January 2020). "Blue magnesium alloy laptops: Premium price, plastic feel, but lightweight". ZDNet, A Ruddy VENTURES Company.
- ^ "Magnesium (Pulverization)". International Plan on Chemic Safety (IPCS). IPCS INCHEM. April 2000. Retrieved 21 Dec 2011.
- ^ Magnesium. Sigma Aldrich
- ^ "Science Safety: Chapter eight". Regime of Manitoba. Retrieved 21 August 2007.
- ^ "Chemical science : Periodic Table : magnesium : chemical reaction data". webelements.com. Retrieved 26 June 2006.
- ^ "The Reaction Between Magnesium and CO2". Purdue Academy. Retrieved 15 June 2016.
- ^ Cote, Arthur Eastward. (2003). Functioning of Fire Protection Systems. Jones & Bartlett Learning. p. 667. ISBN978-0877655848.
- ^ Linsley, Trevor (2011). "Properties of conductors and insulators". Basic Electric Installation Work. p. 362. ISBN978-0080966281.
- ^ Romani, Andrea, M.P. (2013). "Chapter 3. Magnesium in Wellness and Illness". In Astrid Sigel; Helmut Sigel; Roland One thousand. O. Sigel (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 49–79. doi:10.1007/978-94-007-7500-8_3. ISBN978-94-007-7499-5. PMID 24470089.
- ^ "Magnesium in nutrition". MedlinePlus, U.Due south. National Library of Medicine, National Institutes of Wellness. 2 February 2016. Retrieved 13 October 2016.
- ^ Gropper, Sareen S.; Smith, Jack L.; Carr, Timothy P. (v October 2016). Advanced Nutrition and Human Metabolism. Cengage Learning. ISBN978-1-337-51421-7.
- ^ "Vitamins and minerals – Others – NHS Choices". Nhs.uk. 26 November 2012. Retrieved 19 September 2013.
- ^ "Magnesium", pp. 190–249 in "Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride". National Academy Press. 1997.
- ^ Firoz M; Graber M (2001). "Bioavailability of US commercial magnesium preparations". Magnes Res. 14 (4): 257–262. PMID 11794633.
- ^ Lindberg JS; Zobitz MM; Poindexter JR; Pak CY (1990). "Magnesium bioavailability from magnesium citrate and magnesium oxide". J Am Coll Nutr. nine (1): 48–55. doi:10.1080/07315724.1990.10720349. PMID 2407766.
- ^ Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A (April 2000). "Magnesium. An update on physiological, clinical and analytical aspects". Clin Chim Acta. 294 (1–2): 1–26. doi:10.1016/S0009-8981(99)00258-ii. PMID 10727669.
- ^ a b c "Magnesium". Umm.edu. Academy of Maryland Medical Center. 7 May 2013. Archived from the original on xvi February 2017. Retrieved nineteen September 2013.
- ^ Wester PO (1987). "Magnesium". Am. J. Clin. Nutr. 45 (five Suppl): 1305–1312. doi:10.1093/ajcn/45.v.1305. PMID 3578120.
- ^ Arnaud MJ (2008). "Update on the assessment of magnesium condition". Br. J. Nutr. 99 Suppl 3: S24–S36. doi:10.1017/S000711450800682X. PMID 18598586.
- ^ Rob PM; Dick Grand; Bley N; Seyfert T; Brinckmann C; Höllriegel V; et al. (1999). "Can one actually measure out magnesium deficiency using the brusque-term magnesium loading test?". J. Intern. Med. 246 (iv): 373–378. doi:x.1046/j.1365-2796.1999.00580.x. PMID 10583708. S2CID 6734801.
- ^ Franz KB (2004). "A functional biological marking is needed for diagnosing magnesium deficiency". J Am Coll Nutr. 23 (6): 738S–741S. doi:x.1080/07315724.2004.10719418. PMID 15637224. S2CID 37427458.
- ^ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Biomedical Publications. pp. 875–877. ISBN978-0962652370.
- ^ a b c Ayuk J.; Gittoes Northward.J. (March 2014). "Gimmicky view of the clinical relevance of magnesium homeostasis". Register of Clinical Biochemistry. 51 (2): 179–188. doi:10.1177/0004563213517628. PMID 24402002. S2CID 21441840.
- ^ Rosanoff, Andrea; Weaver, Connie M; Rude, Robert K (March 2012). "Suboptimal magnesium condition in the United States: are the wellness consequences underestimated?" (PDF). Nutrition Reviews. seventy (3): 153–164. doi:ten.1111/j.1753-4887.2011.00465.ten. PMID 22364157.
- ^ Geiger H; Wanner C (2012). "Magnesium in disease". Clin Kidney J. 5 (Suppl 1): i25–i38. doi:ten.1093/ndtplus/sfr165. PMC4455821. PMID 26069818.
- ^ Zipes DP; Camm AJ; Borggrefe M; et al. (2012). "ACC/AHA/ESC 2006 Guidelines for Direction of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Expiry: a report of the American College of Cardiology/American Heart Clan Task Force and the European Society of Cardiology Commission for Exercise Guidelines (writing commission to develop Guidelines for Direction of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Center Rhythm Association and the Heart Rhythm Society". Apportionment. 114 (x): e385–e484. doi:10.1161/CIRCULATIONAHA.106.178233. PMID 16935995.
- ^ James MF (2010). "Magnesium in obstetrics". Best Pract Res Clin Obstet Gynaecol. 24 (3): 327–337. doi:10.1016/j.bpobgyn.2009.11.004. PMID 20005782.
- ^ Euser, A. K.; Cipolla, M. J. (2009). "Magnesium Sulfate for the Handling of Eclampsia: A Brief Review". Stroke. xl (4): 1169–1175. doi:10.1161/STROKEAHA.108.527788. PMC2663594. PMID 19211496.
- ^ Giannini, A. J. (1997). Drugs of Abuse (Second ed.). Los Angeles: Physicians Management Data Co. ISBN978-0874894998.
- ^ Teigen L, Boes CJ (2014). "An evidence-based review of oral magnesium supplementation in the preventive treatment of migraine". Cephalalgia (Review). 35 (x): 912–922. doi:10.1177/0333102414564891. PMID 25533715. S2CID 25398410.
There is a strong trunk of evidence demonstrating a relationship between magnesium status and migraine. Magnesium likely plays a role in migraine development at a biochemical level, but the function of oral magnesium supplementation in migraine prophylaxis and treatment remains to exist fully elucidated. The strength of prove supporting oral magnesium supplementation is express at this time.
- ^ Gowariker, Vasant; Krishnamurthy, 5. P.; Gowariker, Sudha; Dhanorkar, Manik; Paranjape, Kalyani (8 April 2009). The Fertilizer Encyclopedia. p. 224. ISBN978-0470431764.
- ^ McGuire, John; Kulkarni, Mona Shah; Baden, Harris (Feb 2000). "Fatal Hypermagnesemia in a Child Treated With Megavitamin/Megamineral Therapy". Pediatrics. 105 (2): E18. doi:x.1542/peds.105.ii.e18. PMID 10654978. Retrieved i February 2017.
- ^ Kontani M; Hara A; Ohta South; Ikeda T (2005). "Hypermagnesemia induced by massive cathartic ingestion in an elderly adult female without pre-existing renal dysfunction". Intern. Med. 44 (5): 448–452. doi:10.2169/internalmedicine.44.448. PMID 15942092.
- ^ Kutsal, Ebru; Aydemir, Cumhur; Eldes, Nilufer; Demirel, Fatma; Polat, Recep; Taspınar, Ozan; Kulah, Eyup (February 2000). "Astringent Hypermagnesemia equally a Event of Excessive Cathartic Ingestion in a Child Without Renal Failure". Pediatrics. 205 (2): 570–572. doi:10.1097/PEC.0b013e31812eef1c. PMID 17726419.
Cited sources
- Rumble, John R., ed. (2018). CRC Handbook of Chemical science and Physics (99th ed.). Boca Raton, FL: CRC Press. ISBN978-1-1385-6163-2.
External links
- Magnesium at The Periodic Table of Videos (University of Nottingham)
- Chemistry in its element podcast (MP3) from the Royal Social club of Chemistry's Chemistry Globe: Magnesium
- "Magnesium – a versatile and often overlooked element: new perspectives with a focus on chronic kidney disease". Clin Kidney J. 5 (Suppl ane). February 2012. Archived from the original on nine June 2013.
Hydrogen Chloride And Ammonia Reaction,
Source: https://en.wikipedia.org/wiki/Magnesium
Posted by: swingleketter.blogspot.com



0 Response to "Hydrogen Chloride And Ammonia Reaction"
Post a Comment