Sodium Hydroxide Dissolving In Water
OVERVIEW
- A 10M sodium hydroxide (NaOH) stock solution is used for many applications including adjusting the pH of various solutions.
- The Normality of NaOH solution is equal to the molarity of the solution. This ways that the normality of a 10M solution of NaOH is equal to 10N.
- The molecular weight of NaOH is 40. That means you need to dissolve 40 g of NaOH in water to obtain a 1 liter of 1M (or 1N) NaOH solution. To prepare a 10M NaOH solution, you need to deliquesce x times more NaOH i.e., 400 g of NaOH for ane L solution.
- Here nosotros volition set up 100 ml of 10M NaOH solution. Therefore, we need to have 40 g of NaOH.
- Dissolution of NaOH is a hyperthermic reaction (generates estrus), therefore, NaOH is added in water in small quantities instead of adding all at a fourth dimension to limit the heat generation every bit excessive heat can harm the container (drinking glass beaker).
Requirements
Reagents and solutions
Sodium hydroxide (NaOH) pallet
Deionized / Double distilled water
Equipment and disposables
Measuring cylinder/volumetric flask
Erlenmeyer flask / Beaker
Glass rod/Magnetic stirrer
Water ice bucket with water ice (optional)
Weighing Residuum
Composition
ten M NaOH solution
Objective
Preparation of 100 ml of 10 M NaOH solution in water
Preparation
Use personal protective equipment (lab glaze, gloves, goggles etc) for your prophylactic and follow the guidelines of your constitute.
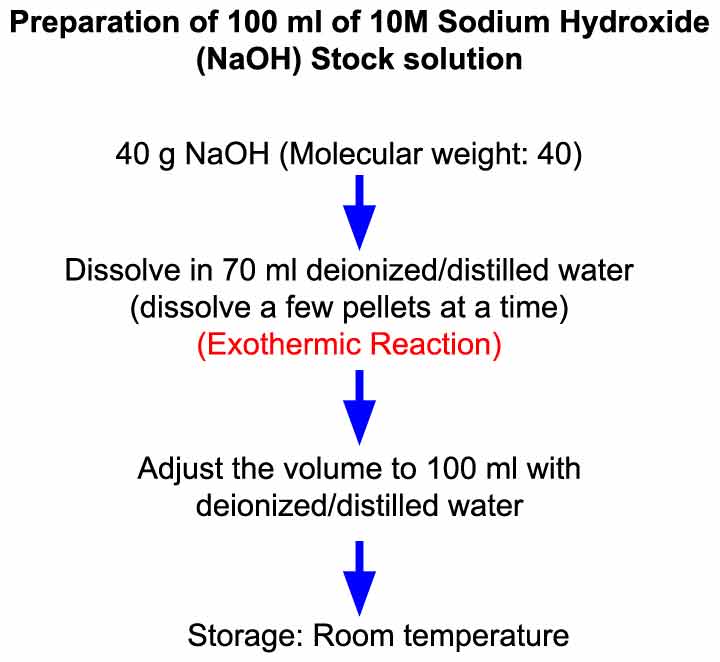
Pace 1: To set up 100 ml of 10 M NaOH solution, weigh out 40 thousand of NaOH (molecular weight: 40).
Precaution:
Don't expose solid NaOH to air for a long fourth dimension while weighing. NaOH is hygroscopic in nature and absorbs h2o from the atmosphere quickly.
Pace 2: Deliquesce NaOH pellets in 70 ml h2o by adding minor quantities of NaOH in the h2o.
◊ Take 70 ml deionized/double distilled water in a beaker.
◊ Add a few pellets (4 – 5 pellets each time) of NaOH in water and await until they dissolve. Repeat this until all 40 thousand NaOH pellets are dissolved in water.
Precautions:
i. The dissolution of NaOH is a highly exothermic reaction, which can even pause the glass beakers. It is recommended to continue the beaker on ice while dissolving NaOH.
2. Never add together 70 ml water to 40 g NaOH of pellet.
Step three: Accommodate the solution volume to 100 ml with deionized/double distilled water.
◊ Transfer solution to a measuring cylinder/volumetric flask (capacity: 100 ml) and suit the volume to 100 ml with deionized/double distilled water.
Storage:
The solution can be stored at room temperature for several months.
Note:
At that place is no need to sterilize this solution past autoclaving. No microorganism will abound in concentrated NaOH solution.
Applications
● NaOH is a strong base and commonly used to adjust the pH of many solutions.
● Plasmid isolation by alkaline lysis method
● Training of EDTA solution
| Follow the table to set up NaOH solution of specific concentration and volume | ||||
| Conc. / Book | 50 ml | 100 ml | 250 ml | 500 ml |
| 1 M | 2.00 g | four.00 g | 10.00 g | 20.00 one thousand |
| two M | 4.00 1000 | 8.00 thou | xx.00 g | 40.00 yard |
| 5 Yard | ten.00 1000 | 20.00 1000 | 50.00 one thousand | 100.00 1000 |
| 10 M | 20.00 g | 40.00 thousand | 100.00 g | 200.00 yard |
CALCULATOR
Apply figurer to calculate the quantities of NaOH for the preparation of NaOH solution of specific concentration and book
Molecular weight of NaOH: 40
Volume of Sodium hydroxide solution: ml
(change the book of the solution)
Molarity of Sodium hydroxide solution: One thousand
(Change the molarity of the solution)
Amount of sodium hydroxide: m
Was this mail service helpful?
Sodium Hydroxide Dissolving In Water,
Source: https://www.laboratorynotes.com/preparation-of-10-m-sodium-hydroxide-naoh-solution/
Posted by: swingleketter.blogspot.com


0 Response to "Sodium Hydroxide Dissolving In Water"
Post a Comment